It is also called Vitamin B3, Vitamin PP, white granular powder or white crystalline powder, odorless or almost odorless, bitter taste. It is mainly used in Vitamin medicine, food additive, feed additive, cosmetics additives. It can also be used for synthesis of other chemical products.
[CHEMICAL NAME] 3-Pyridinecarboxamide
[ENGLISH NAME] Nicotinamide/Niacinamide
[SYNONYM] Vitamin B3,Vitamin PP
[CAS#] 98-92-0
[MOLECULAR FORMULA AND WEIGHT] C6H6N2O=122.13
[STRUCTURAL FORMULA]
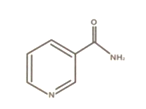
[CHARACTERISTICS] White granular powder or white crystalline powder, odorless or almost odorless,
bitter taste
[PHYSICAL PROPERTIES] Melting Point:128~131℃
Boiling Point:150~160℃(0.07kPa)
Flash Point:182℃
Solubility:Soluble in water, ethanol, sodium carbonate solution, sodium
hydroxide solution, dissolving in glycerin, almost insoluble in ether
[MAIN USES] Vitamin medicine, food additive, feed additive, cosmetics additives. It can also
be used for synthesis of other chemical products
[PRODUCT GRADES] Pharmaceutical Grade, Food Grade, Cosmetic Grade, Feed Grade
[EXECUTIVE STANDARD] The products of our company comply with standards USP, EP, BP, JP, ChP,
GB/T7301-2002, etc.
[PACKING] 20KG/Carton, 20KG/Bag,25KG/Carton,1000Kg/Bag
[STORAGE] Dry, cool, indoor storage
The Nicotinamide of Redpont Biotech has the following characteristics:
1. High starting point.
The production process is designed and constructed strictly according to GMP requirements. The product specifications include pharmaceutical grade, food grade, feed grade, cosmetic grade. The quality conforms to the the United States Pharmacopoeia(USP), the European Pharmacopoeia (EP), the British Pharmacopoeia(BP), the Japanese Pharmacopoeia(JP), the Chinese Pharmacopoeia, the Chinese feed standard GB/T7301-2002,etc.
2. Advanced equipments and high degree automation.
The most advanced equipments have been used in the production, and the process is integrated controlled by the DCS, with continuous production, simple operation, fewer personnel, which greatly ensure the stability of product’s quality.
3. High safety performance of the production process.
Strict security risk assessment of the whole production process has been made; The investment for links which may cause safety accidents has been increased to achieve one key to stop design, ensures safety production.
4. Well-equipped facilities of energy saving and environmental protection.
The equipments have been increased to make use of the heat generated in the production process, to recycle the waste steam, which greatly saved energy. The waste water, waste gas and waste residue produced in the process have been discharged up to the standards.
5.Strong awareness of food safety.
Besides strictly in accordance with the requirements of GMP management on production process, a set of product traceability system has been brought in. That is to say every unit (box or bag) product has an unique bar code identification, which can track all the situation during product’s manufacturing process. Customers can also use this bar code to carry on production management.
6. Use robots to reduce labor intensity.
Humanized management has been taken on the production process. The robot operation has been used in the high intensity labor field, such as product’s transfering, stacking, which greatly protects the operators.
Automated warehouse, saving time and effort, high efficiency and energy saving.

Product Package

